We are currently developing several research axes:
1. Characterizing oncogenesis mechanisms and developing novel therapeutic approaches for NK/T lymphoma
Extranodal nasal-type NK/T lymphomas are rare and aggressive peripheral T-cell lymphomas associated with Epstein-Barr virus (EBV), whose oncogenesis remains poorly understood.
Our research on NK/T lymphoma is based on close collaboration with Prof. Hermine and Prof. Jaccard, clinical experts in this pathology. New cases are identified through a national Multidisciplinary Team Meeting dedicated to NK/T lymphomas, in which Lucile Couronné actively participates. The team also works closely with the LYSATOMIC network of the LYSA cooperative group to centralize patient samples and collect associated clinical data.
Investigating Tumor Escape Mechanisms in NK/T Lymphoma
While EBV infection in B cells can lead to uncontrolled proliferation and lymphoma development in immunocompromised individuals, NK/T lymphoma patients typically do not present with primary immunodeficiency. This suggests other mechanisms of immune escape are involved in NK/T lymphomagenesis. Reported mechanisms include overexpression of the immune checkpoint PD-L1 by NK/T lymphoma cells and mutations in genes involved in immune surveillance.
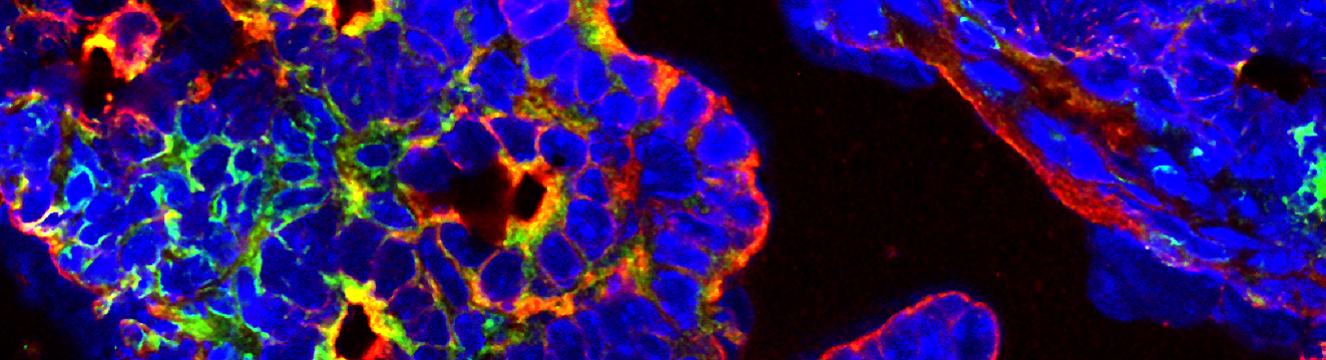
Our team aims to unravel both intrinsic and extrinsic mechanisms associated with tumor escape in NK/T lymphomas. To achieve this, we use in vitro and in vivo immune response models based on cell lines and/or primary patient cells. We also analyze the tumor microenvironment in NK/T lymphoma samples using advanced techniques such as multiplex immunofluorescence and spatial transcriptomics.
Identifying Predictive Factors for Immunotherapy Response in NK/T lymphoma
PD-1 inhibitors (aPD1) have shown promising, though partial, efficacy in improving anti-tumor immunity in relapsed/refractory and advanced-stage patients. We aim to identify predictive biomarkers of aPD1 response through multi-omics profiling of tumor samples using cutting-edge techniques, including next-generation sequencing, optical genome mapping, multiplex immunofluorescence, spatial transcriptomics, and methylome analysis. In addition, we analyze patients’ immune status using mass cytometry, single-cell RNA sequencing and T-cell repertoire analysis to obtain a comprehensive view of immune responses.
Developing Novel Therapeutic Approaches in NK/T lymphoma
The prognosis for relapsed/refractory and disseminated forms remains poor. We aim to develop novel therapeutic approaches, either as monotherapies or in combination, with a focus on immunomodulatory therapies and those synergizing with aPD1. To this end, we collaborate with industry partners to evaluate promising new molecules. Additionally, we are working to create innovative preclinical models, including mouse and non-mouse (organoid) models, to assess therapeutic efficacy in relevant experimental contexts.
2. Investigating the Pathophysiology of Indolent T-cell Lymphoproliferations of the Digestive Tract
Indolent T-cell lymphoma of the gastrointestinal tract is a very rare disease (fewer than 70 reported cases) characterized by multifocal lesions in the digestive tract, mainly in the small intestine and colon. It involves clonal infiltration of the epithelium and lamina propria by small CD4+ T cells. While generally indolent, it can cause persistent digestive symptoms and quality-of-life impairments, with occasional progression to aggressive lymphoma years after diagnosis. Diagnosis is challenging, and no effective standard therapy exists.
Due to its rarity in humans, we studied a spontaneous feline model, low-grade intestinal T cell lymphoma (LGITL )in collaboration with the National Veterinary School of Alfort (Dr. Valérie Freiche). Our data indicate that LGITL shares striking similarities with human GI-TLPD, validating its relevance as a model for human disease. Moreover, we identified deregulation of the JAK/STAT pathway (Phospho-STAT5 overexpression) in LGITL, which may represent a therapeutic target common to both species.
Given the emerging nature of this feline entity, studying T-LGIL could offer valuable insights into characterizing the human disease, particularly for testing JAK/STAT pathway inhibitors. We conducted pan-genomic and transcriptomic analyses on 20 cats with LGITL. Currently, we are performing functional studies to explore the role of candidate genes in lymphomagenesis and assess their recurrence in humans.
3. Molecular Characterization of B-cell Lymphomas Associated with Immune Deficiencies
Inborn errors of immunity (IEIs) represent a heterogeneous group of diseases often diagnosed in childhood, predisposing patients to lymphoma development. The genetic landscape of lymphomas associated with IEIs is poorly understood, and treatments are not standardized, posing challenges due to the increased toxicity of chemotherapy in these patients. Furthermore, lymphoma may be the first manifestation of an undiagnosed underlying IEI.
We aim to molecularly characterize lymphomas associated with IEIs. In collaboration with the pediatric immuno-hematology and adult hematology departments at Necker Hospital and its pathology department, we will analyze a cohort of approximately 200 B-cell lymphomas (both pediatric and adult) with well-defined IEIs. This study will use integrative pan-omics approaches to identify signatures specific to various IEIs types.
Comparative analyses with lymphomas in immunocompetent patients will also be conducted to identify clinical, biological, histological, and molecular biomarkers indicating underlying IEIs.
In parallel, we aim to explore the genetic landscape of pediatric B-cell lymphomas occurring post-transplantation in acquired immunodeficiency settings. This study seeks to elucidate the pathogenesis of these lymphomas and identify prognostic or predictive treatment-response factors to improve therapeutic management.
Molecular characterization of lymphomas associated with immune deficiencies—whether primary or acquired—will not only shed light on lymphomagenesis mechanisms in immunodeficient contexts but also enhance our understanding of lymphomas in immunocompetent patients, revealing potential innovative therapeutic targets.