Published on
The causes of ALS are considered to be multifactorial. It affects both sexes, and its incidence increases with time from the age of 40.
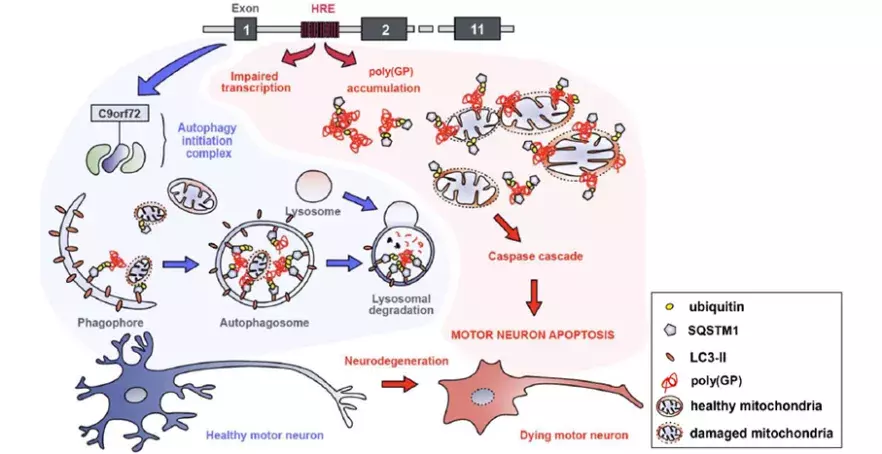
Amyotrophic lateral sclerosis (ALS), also known as Charcot's disease, is a devastating neurodegenerative disease characterised by the loss of cortical and spinal motor neurons. The most frequent known genetic cause of this disease is the expansion of the number of repeats of a group of 6 nucleotides, the ‘bases’ of DNA, in a non-coding part of the C9orf72 gene.
This expansion is associated with both reduced levels of C9orf72 protein expression and the production of dipeptide repeats (two amino acids), particularly poly(Glycine-Proline) (poly(GP)), which can be detected in patients' cerebrospinal fluid (making it a candidate biomarker for detecting and diagnosing ALS of C9orf72 genetic origin).
In order to gain a better understanding of the cellular mechanisms affected by this nucleotide repeat, the team from the Translational Research for Neurological Disorders laboratory, led by Edor Kabashi at Institut Imagine (Inserm, AP-HP, Université Paris Cité), developed a zebrafish model in which they were able to reproduce this genetic feature and the characteristics of the disease. By investigating the pathological mechanisms involved, they demonstrated that the reduction in the level of the C9orf72 protein and the expression of poly(GP) act in synergy to induce the degeneration of motor neurons, leading to paralysis. They observed that poly(GP) accumulate in motor neurons in particular, due to a defect in autophagy, a mechanism involved in the degradation of cellular components.
The project also benefited from collaboration with Chiara Guerrera’s team and Nicolas Goudin from the SFR Necker. By purifying zebrafish motor neurons and analysing their proteome (all the proteins in the cell), the team confirmed the direct involvement of mitochondria in the pathogenicity of ALS associated with the C9orf72 gene mutation. By reconstructing motor neuron structures in 3D, they demonstrated that poly(GP)s accumulate in the mitochondria, causing abnormalities in their morphology. In a healthy motor neuron, damaged mitochondria are degraded by the cell via the autophagy pathway, in a mechanism known as mitophagy. For the first time, researchers have demonstrated a direct link between mutation of the C9orf72 gene and a defect in mitophagy in motor neurons. The reduction in autophagy and mitophagy mechanisms leads to the accumulation of poly(GP) and defective mitochondria in motor neurons, which activate the signals triggering apoptosis, the cell death of these motor neurons.
The team was also able to show that autophagy and mitophagy could be restored after treating fish embryos with molecules that stimulate these pathways. The development of this ALS model in zebrafish will therefore make it possible to test new drug candidates to improve both autophagy and reduce poly(GP) and abnormal mitochondria accumulation, in the hope of identifying a suitable treatment for ALS patients.
Reference :
Poly-GP accumulation due to C9orf72 loss of function induces motor neuron apoptosis through autophagy and mitophagy defects
H de Calbiac et al., Autophagy, 2024
Corresponding author : Hortense de Calbiac