Publish at
Presentation
Nadine Cerf-Bensussan, MD PhD, is a Research Director at the French National Institute of Health and Medical Research (INSERM), and head of the Laboratory of Intestinal Immunity at Imagine Institute and Paris University Paris. She was awarded in 2014 the Research Prize of INSERM and an ERC advanced grant, in 2017 the Distinguished Scientific Achievement Award from the Society for Mucosal Immunology and in 2018 the Maki Celiac Disease Tampere Prize.
INTESTINAL IMMUNITY
With a surface estimated between 35-70m2, the intestine is the main body’s interface. It forms a highly dynamic and tightly regulated barrier that is crucial to absorb nutrients and fuel our metabolic needs, but also to control the vast and complex community of microbes, which colonizes this surface after birth. Our main objective is to dissect how epithelial cells cooperate with immune cells to restrict body access to microbes and undigested food antigens while avoiding deleterious inflammation and tissue damage. In parallel, we analyse the mechanisms by which the Segmented Filamentous Bacterium orchestrates the post-natal maturation of the gut immune barrier.
Pathogenesis of severe human enteropathies
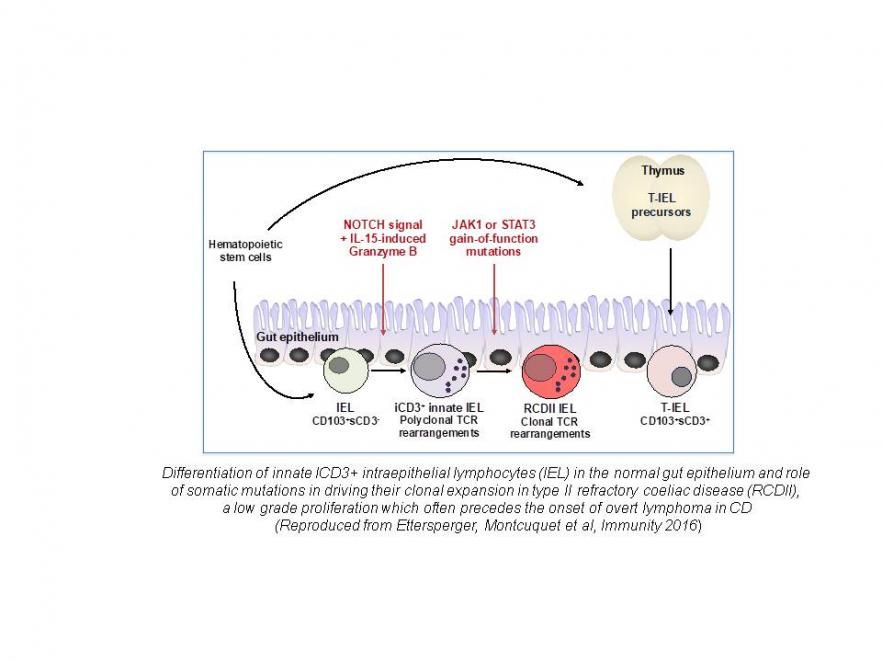
1. Coeliac disease (CD): is a long-standing axis of research for our team, which coordinates a National INCA network for diagnosis and treatment of complicated CD. This frequent autoimmune-like disease is driven by chronic ingestion of wheat-derived gluten in genetically predisposed individuals. . Based on the analysis of a large cohort of well-characterized patients, on in situ and ex vivo analyses of human intestinal lymphocytes and on mouse models, we investigate the mechanisms that drive intestinal tissue damage and lymphomagenesis. We have shown that interleukin 15 (IL-15) is a key player. This cytokine impairs local immunoregulation and cooperates with antigen-specific CD4+ T cells to activate cytotoxic lymphocytes and tissue damage. IL-15 can also promote the onset of lymphomas from an unusual subset of lymphocytes that we have identified in the gut epithelium. The latter lymphocytes differentiate locally from bone marrow-derived precursors in response to sequential NOTCH and IL-15 signals. NOTCH signals initiate T cell differentiation, revealed by expression of intracellular CD3 (iCD+) and evidence of T cell receptor rearrangements. IL-15 induces Granzyme B, a protease that cleaves NOTCH into a peptide deprived of transcriptional activity. As a consequence, T cell differentiation is prematurely stopped. Cells are redirected by default toward the NK pathway and acquire NK markers and function. While innate-like iCD3+ lymphocytes form a minor polyclonal subset of lymphocytes in the normal adult gut epithelium, their massive clonal expansion is a diagnosis hallmark of CD-associated lymphomas. Strikingly, in many cases, malignant innate-like T cells display gain of function somatic mutations in JAK1 and or STAT3, which confer a selective advantage in the cytokine (IL-15-) rich environment of the coeliac intestine. Our present work aims at further dissecting the mechanisms that drive malignant transformation and progression from low- to high-grade lymphomas and at using these results to improve diagnostic and therapeutic strategies.
2. Mendelian inherited intestinal disorders: This more recent axis of research is currently supported by an Advanced ERC grant and has two complementary goals: - to take advantage of monogenic diseases to identify non redundant mechanisms indispensable to sustain the human gut barrier; 2- to set up a diagnosis platform and improve the care of these rare but life-threatening diseases. Patients are studied in collaboration with clinicians in France (Immunobiota protocol) and in Europe (GENIUS network). The molecular defect is searched for by combining phenotype-based functional analyses, custom made targeted gene panel sequencing and whole exome sequencing. . Novel mutations in the IL-10 receptor causing very early onset colitis have been found, including one with a founder effect as well as mutations in MALT1 as a cause of autoimmune enteropathy combined with severe immunodeficiency. Biallelic mutations in the epithelial NADPH oxidase DUOX2 and in the intestinal alkaline phosphatase have been identified as novel genetic causes cause of early onset colitis. Several novel candidate genes are under study and highlight the cooperative role of the epithelial or the hematopoietic components of the gut barrier in intestinal homeostasis. In parallel to this work, an H2020 program coordinated by F. Ruemmele aims at assessing and improving therapy of pediatric inflammatory bowel diseases.
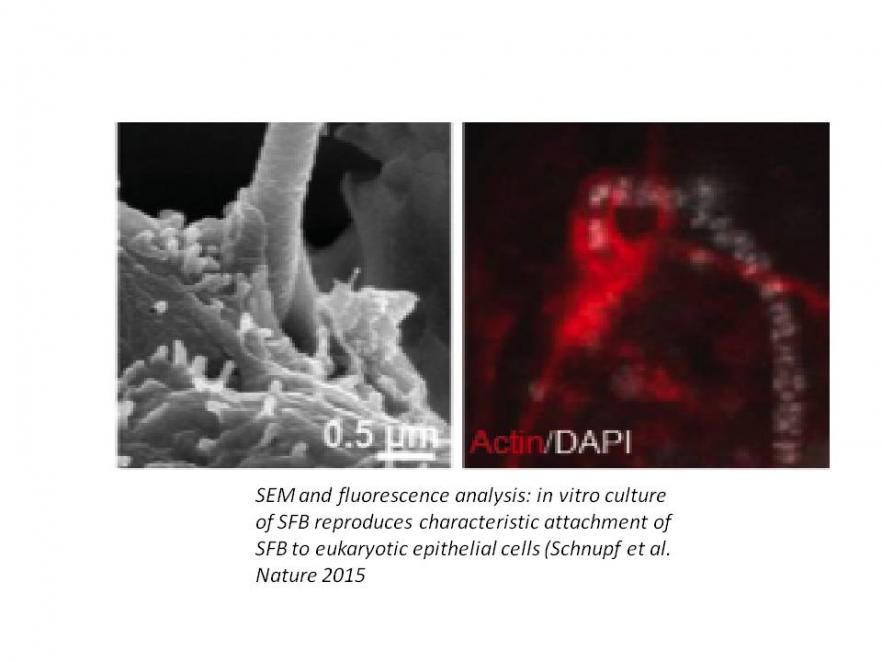
2- Host-microbiota cross-talk and development of the intestinal barrierThe gut microbiota has emerged as a key determinant in health and disease. One outstanding challenge is now to identify which members of the microbiota are indispensable to promote host fitness and to delineate their mechanisms of action. Based on analyses led in gnotobiotic mice, we have demonstrated that Segmented Filamentous Bacterium (SFB) is indispensable to drive the post-natal maturation of the mouse gut immune barrier. SFB is notably a potent inducer of secondary and tertiary gut lymphoid tissue. It strongly stimulates IgA secretion and intestinal T cells and, most strikingly, is indispensable to launch gut homeostatic TH17 responses. Unlike other commensals, which typically reside in the intestinal lumen and have limited access to the intestinal surface, SFB is unique in that it intimately attaches to ileal epithelial cells. This attachment is species-specific, suggesting a co-evolutionary symbiosis between SFB and its many vertebrate hosts. Based on the hypothesis that SFB growth requires direct contact with live epithelial cells, we have developed a method for in vitro culture of SFB, which allows to recapitulate its very characteristic life and to induce a transcriptomic epithelial response similar to that observed in vivo. Thus, attachment allows SFB access to host resources indispensable for its growth. Conversely, colonization of SFB does not lead to pathology but induces as yet poorly defined signals, which stimulate innate and adaptive immunity. Our goals are now to Identify the mechanisms of SFB stimulation at the cellular and molecular levels, to define whether SFB is part of the human microbiota and if so, to define its impact on the intestinal immune barrier in health and diseases with the long term goal to develop SFB into a probiotic and or a vaccinal platform.
Team
Resources & publications
-
 2019Journal (source)Nat Microbiol
2019Journal (source)Nat MicrobiolIntracellular offspring released from SFB filaments are flagellated.
-
 2019Journal (source)J Crohns Colitis
2019Journal (source)J Crohns ColitisDiagnostic Yield of Next-Generation Sequencing in Very Early-Onset Inflammato...
-
 2019Journal (source)Immunity
2019Journal (source)ImmunityScratching Beneath the Surface: Linking Skin Pathology with Food Allergy.
-
 2019Journal (source)Gut
2019Journal (source)GutNKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointe...
-
 2019Journal (source)Curr. Opin. Immunol.
2019Journal (source)Curr. Opin. Immunol.Modulation of the gut microbiota to improve innate resistance.
-
 2019Journal (source)F1000Res
2019Journal (source)F1000ResRecent advances in celiac disease and refractory celiac disease.
-
 2019Journal (source)Gastroenterology
2019Journal (source)GastroenterologyEfficacy of Ruxolitinib Therapy in a Patient With Severe Enterocolitis Associ...
-
 2019Journal (source)EMBO Mol Med
2019Journal (source)EMBO Mol MedHuman ALPI deficiency causes inflammatory bowel disease and highlights a key ...
-
 2017Journal (source)Immunity
2017Journal (source)ImmunityInterleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Devel...
-
 2015Journal (source)Nature
2015Journal (source)NatureGrowth and host interaction of mouse segmented filamentous bacteria in vitro.
-
 2014Journal (source)Immunity
2014Journal (source)ImmunitySegmented filamentous bacterium uses secondary and tertiary lymphoid tissues ...