Published on 10.12.2025
Presentation
Cognitive functions depend on the precise construction of complex neural circuits which begins during early embryonic development. Studies in the past decades have revealed that abnormal brain development participates to the aetiology of multiple neurological and psychiatric disorders including epilepsy, schizophrenia, autism spectrum disorders, obsessive-compulsive behaviors and bipolar disorders.
Our work has shown that proper cortical development also depends on the action of different cell types that are transiently present during the construction of neural circuits. These transient signalling neurons express at high levels genes whose mutations have been associated with neurological and psychiatric disorders. At the earliest stages of corticogenesis in mice, long before any functional synapses are formed in the cerebral cortex, these neurons express genes that are involved in neurotransmission and are thought to be exclusively present at mature synapses. We have published and unpublished data showing that “synaptic” genes, whose mutations have been associated with pathological conditions, control neuronal migration during embryogenesis. Our recent results in primates also suggest that an increase in both number and diversity of migrating transient signalling neurons could be an evolutionary addition to wire higher-order cortical areas in the cerebral cortex and to increase vertebrate brain complexity and cognitive function.
Our data show that transient variations in the kinetics of arrival of these migrating signalling neurons during early development, or of their death at the end of corticogenesis have profound consequences on the construction of normal and pathological neural circuits. We have shown that changes in neuronal migration during embryonic life lead to dysfunctional cortical circuits spanning from severe neonatal cortical malformations to subtle and transient defects, which mimics diseases with onset at puberty/adolescence. By coupling studies on the function and dysfunction of transient neuron development in mice and primates, our future projects aim at linking developmental neuroscience with evolution and pathology in humans.
Our projects span from early onset cortical malformations to susceptibility to later-onset diseases characteristic of psychiatric illnesses. They are now reaching the stage where we wish to, and can, ask questions relevant to human health. Thus, we have decided to join the Institute Imagine (Institut des Maladies Génétiques, Hôpital Necker Enfants malades, Paris) and the Institute of Psychiatry and Neurosciences of Paris (IPNP, Hôpital St Anne, Paris) to be able to develop this translational project in collaboration with neuroscientists, human geneticists and clinicians. Our Team moved in September 2017 and is reinforced by 4 people holding permanent positions (two researchers, one Engineer and one MD). This allows closer interactions with human geneticists and clinicical experts in rare diseases, brain imaging and malformations. Our team’s strong expertise in cortical development will introduce a novel dimension fostering synergistic interactions across disciplinary boundaries. Our future projects should provide new genetic tools to develop mouse models for cortical abnormalities and contribute to the understanding and diagnosis of neurodevelopmental diseases in humans.
Team
Resources & publications
-
 2025Journal (source)Development
2025Journal (source)DevelopmentDifferential contribution of P73+ Cajal-Retzius cells and Reelin to cortical ...
-
 2024Journal (source)J Clin Invest
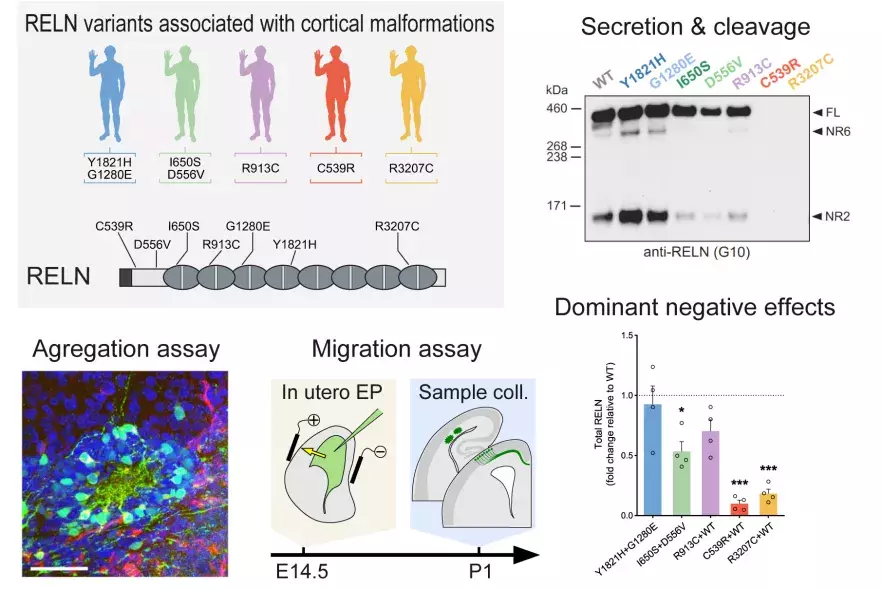
2024Journal (source)J Clin InvestDe novo monoallelic Reelin missense variants act in a dominant-negative manne...
-
 2023Journal (source)Dev Cell
2023Journal (source)Dev CellRepurposing of the multiciliation gene regulatory network in fate specificati...
-
 2023Journal (source)J Comp Neurol
2023Journal (source)J Comp NeurolDiversity within olfactory sensory derivatives revealed by the contribution o...
-
 2023Journal (source)Cell
2023Journal (source)CellTransient perinatal metabolic shifts determine neuronal survival and functio...
-
 2023Journal (source)Nat Commun
2023Journal (source)Nat CommunAberrant survival of hippocampal Cajal-Retzius cells leads to memory deficits...
-
 2023Journal (source)Int J Mol Sci
2023Journal (source)Int J Mol SciActivation of the PI3K/AKT/mTOR Pathway in Cajal-Retzius Cells Leads to Their...
-
 2023Journal (source)Curr Opin Neurobiol
2023Journal (source)Curr Opin NeurobiolCajal-retzius cells: Recent advances in identity and function
-
 2021Journal (source)Development
2021Journal (source)DevelopmentSingle-cell transcriptomics of the early developing mouse cerebral cortex dis...
-
 2021Journal (source)Development
2021Journal (source)DevelopmentThe multiple facets of Cajal-Retzius neurons.
-
 2021Journal (source)Semin Cell Dev Biol
2021Journal (source)Semin Cell Dev BiolWiring of higher-order cortical areas: Spatiotemporal development of cortical...
-
 2020Journal (source)Cell Rep
2020Journal (source)Cell RepEvolutionary Gain of Dbx1 Expression Drives Subplate Identity in the Cerebral...
-
 2020Journal (source)Nat Commun
2020Journal (source)Nat CommunDevelopmental cell death regulates lineage-related interneuron-oligodendrogli...
-
 2020Journal (source)Front Cell Dev Biol
2020Journal (source)Front Cell Dev BiolHow Do Electric Fields Coordinate Neuronal Migration and Maturation in the De...
-
 2019Journal (source)Neurol Genet
2019Journal (source)Neurol GenetDelineating FOXG1 syndrome: From congenital microcephaly to hyperkinetic ence...
-
 2019Journal (source)Cereb. Cortex
2019Journal (source)Cereb. CortexExtracellular Pax6 Regulates Tangential Cajal-Retzius Cell Migration in the D...
-
 2019Journal (source)Eur J Med Genet
2019Journal (source)Eur J Med GenetMutations in TBR1 gene leads to cortical malformations and intellectual disab...
-
 2018Journal (source)Curr. Opin. Neurobiol.
2018Journal (source)Curr. Opin. Neurobiol.Cortical developmental death: selected to survive or fated to die.
-
 2018Journal (source)Cereb. Cortex
2018Journal (source)Cereb. CortexEnhanced Abventricular Proliferation Compensates Cell Death in the Embryonic ...
-
 2017Journal (source)Cell Rep
2017Journal (source)Cell RepTargeted Inactivation of Bax Reveals a Subtype-Specific Mechanism of Cajal-Re...

Key numbers
22 members
7 nationalities
2 associated labs