Published on 30.07.2025
Collectively, congenital heart diseases are common, affecting 1% of births, but individually they can be rare disorders requiring specific diagnosis and follow-up. Currently, most malformations of the heart structure have an unknown cause.
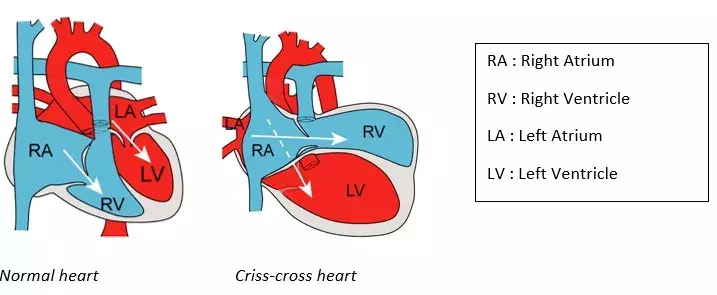
Criss-cross heart is an example of a very rare cardiac malformation (frequency of 1/125,000 births), the genetic cause and embryonic mechanisms of which were unknown. This condition is defined by abnormal connections between atria and ventricles, resulting in crossing of the left and right blood flows on cardiac ultrasound. Criss-cross heart is almost always associated with other cardiac anomalies, such as abnormal connections of the heart to the arteries, or an abnormal position of the ventricles.
The mechanism of criss-cross heart development was still unexplained. Ségolène Bernheim, a cardiac paediatrician and PhD student in the multidisciplinary “Heart Morphogenesis” team led by Sigolène Meilhac at Institut Imagine (Inserm, AP-HP, Université Paris Cité) and Institut Pasteur, has identified the first model of the disease: a mouse line with a mutation in Greb1l, a gene previously associated with kidney malformations. By studying embryonic development daily, Ségolène Bernheim identified the stage at which heart formation becomes abnormal: the embryonic heart unexpectedly tilts at 90° compared with a normal heart.
The team then elucidated the cause of this abnormal tilt: growth of the heart is arrested because there is a shortage of cardiac cells. In the absence of Greb1l, this growth defect generates abnormal mechanical constraints, which impact the shape of the heart: defective position of cardiac valves and ventricles.
The function of the Greb1l gene was unknown. Using large-scale molecular analyses, the team finally linked the function of Greb1l to two cellular mechanisms: first, this gene plays a crucial role in ribosome formation, the cell protein-making machinery; second, Greb1l controls the appearance of cardiac cells, through the process of cell differentiation.
In the absence of Greb1l, these two cellular mechanisms are strongly affected and disrupt the correct embryonic development of the heart. Based on the work of Sigolène Meilhac's team the mechanisms by which the criss-cross heart malformation occurs during pregnancy can be reconstructed. This work leveraged synergy between the fundamental research carried out at Institut Imagine and the clinical and genetic research carried out on AP-HP patients at the hospital Necker-Enfants Malades. It thus contributes directly to better care for patients and information for their families.
The GREB1L gene is known to be involved in other human diseases: kidney malformations, hearing impairments, skeletal or genital anomalies. The cellular mechanisms revealed in this study of cardiac cells will be useful to better understand the origin of diseases in other organs associated with GREB1L.